1911 – Manchester, England
‘The atom contains a core or nucleus of very high density and very concentrated positive charge. Most of the atom is empty space, with the electrons moving about the tiny central nucleus’

ERNEST RUTHERFORD
Working under JJ THOMSON (1856-1940) at the Cambridge Cavendish Laboratory and later at the McGill University in Montreal, in 1898 Rutherford put forward his observation that radioactive elements give off at least two types of ray with distinct properties, ‘alpha’ and ‘beta’ rays.
In 1900 he confirmed the existence of ‘gamma’ rays, which remained unaffected by a magnetic force, whilst alpha and beta rays were both deflected in different directions by such an influence. Although both displayed the ability to stab through solid matter, alpha rays were far less penetrating than beta rays.
He proved through experimental results that they were helium atoms missing two electrons.

Alpha Beta Particles, Gamma Rays in a Magnetic Field
Alpha rays are in fact positively charged helium atoms that become true helium when they slow down and their charge is neutralised by picking up electrons.
Beta rays were later shown to be made up of electrons, and gamma rays to have a shorter wavelength than X-rays.

Alpha Beta Gamma radiation
In Montreal, Rutherford worked with Frederick Soddy and showed that over a period of time, half of the atoms of a radioactive substance could disintegrate. During the process the substance spontaneously transmuted to other elements. During radioactive decay, one kind of atom (radium) was ejecting another kind of atom (helium).
Working with other elements, Rutherford and Soddy found that each radioactive element had its own characteristic ‘half-life’. After one half-life, a sample retained only half its original radioactivity, after two half-lives a quarter, after three half-lives an eighth. The half-life of thorium emanation, now known as radon, was close to a minute. The half-lives of other radioactive elements ranged from a split-second to many billions of years. That of radium was 1620 years, while uranium had a half-life of 4.5 billion years.
The concept of half-life provides a way of measuring the age of rocks. As radioactive atoms decay they emit alpha particles. As these are essentially helium atoms, the amount of helium gas accumulates within the pores and fissures of a sample of a uranium mineral as a measure of how many atoms have decayed. Heating samples to drive off their helium and measuring the amount gives an indication of their age.
In order to provide more reliable dates, measuring the amount of lead, the ultimate decay product, compared with the amount of uranium, eliminates the errors introduced by the escape of some of the helium decay product to the air.
Dating rocks in this way gives an estimate of the age of the Earth, and by implication also the Sun, of around 4.5 billion years.
A radioactive atom is simply a heavy atom, which happens to be unstable. Eventually it disintegrates by expelling an alpha, beta or gamma ray. What remains is an atom of a slightly lighter element. A radioactive atom may decay more than once. Uranium, for instance, transforms itself into a succession of lighter and lighter atoms, one of which is radium, until it achieves stability as a non-radioactive atom of lead.

Working with HANS GEIGER (1882-1945), Rutherford developed the Geiger counter at Manchester University in 1908. This device measured radiation and was used in Rutherford’s work on identifying the make-up of alpha rays.
While he was at McGill, Rutherford had experimented firing alpha particles at a photographic plate. He had noticed that, while the image produced was sharp; if he passed the alpha particles through thin plates of mica, the resulting image on the photographic plate was diffuse. The particles were clearly being deflected through small angles as they passed close to the atoms of mica.
In 1910 his team undertook work to examine the results of directing a stream of alpha particles at a piece of platinum foil. While most passed through, about one in eight thousand bounced back – that is, deflected through an angle of more than 90 degrees.

Deflection of alpha Particles by Thin Metal Foil
In 1911 he put forward the theory that the reason for the rate of deflection was because atoms contained a minute nucleus that bore most of the weight, while the rest of the atom was largely ’empty space’ in which electrons orbited the nucleus much as planets orbit the Sun. The reason that one in eight thousand alpha particles bounced back was because they were striking the positively charged nucleus of an atom, whereas the rest simply passed through the spacious part.
But what was an atomic nucleus made of?
At 100,000th the size of the atom, it would take decades of painstaking experiments to discover.
In 1919, working in collaboration with other scientists, Rutherford artificially induced the disintegration of atoms by collision with alpha particles. In the process the atomic make-up of the element changed as protons were forced out of the nucleus. He transmuted nitrogen into oxygen (and hydrogen) and went on to repeat the process with other elements.
(image source)


 TIMELINE
TIMELINE
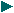 THE ATOM
THE ATOM
<< top of page



 TIMELINE
TIMELINE


































